chloride lewis dot structure|Lewis Dot Structures : Tagatay Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. Mega Rayquaza will be featured in a makeup event globally as a fix for the errors joining the raids experienced in its Elite Raid event on June 29th. In a tweet/X posted on 11th July, @NianticHelp said: Trainers, due to issues affecting Elite Raids featuring Mega Rayquaza, a special global makeup event will be held for all Trainers on Saturday .
PH0 · Lewis Structure Finder
PH1 · Lewis Electron Dot Structures
PH2 · Lewis Dot Structures
PH3 · Lewis Dot Structure for Chlorine Atom (Cl)
PH4 · Lewis Dot Structure
PH5 · How to Draw the Lewis Dot Structure for Cl
PH6 · Drawing Lewis Structures
PH7 · 9.3: Drawing Lewis Structures
PH8 · 7.3 Lewis Symbols and Structures
PH9 · 4.1: Lewis Electron Dot Structures
Find out today's non-runners across today's horse racing fixtures, updated daily by our team of expert racing editors.
chloride lewis dot structure*******Set 13, 2013 — A step-by-step explanation of how to draw the Lewis dot structure for Cl (Chlorine). I show you where Chlorine is on the periodic table and how to determine.Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.Okt 29, 2021 — To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one .chloride lewis dot structure Lewis Dot Structures What is the Lewis electron-dot structure for a molecule of hydrogen chloride? How do you use an electron dot diagram to represent a molecule that has a polar covalent bond? What is the Lewis electron-dot diagram for a fluoride ion?Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the .For example, hydrogen and chlorine each need one more electron to achieve a noble gas configuration. By sharing valence electrons (each atom donates one), the stable HCl HCl molecule is formed. We will use a simplified representation .
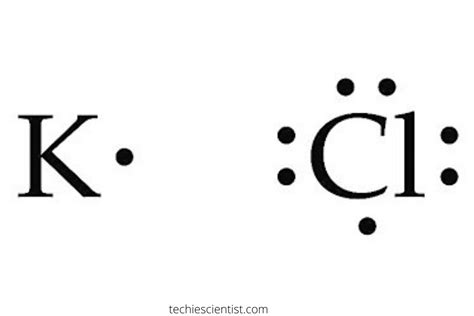
Ene 30, 2023 — Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess .chloride lewis dot structureEne 30, 2023 — Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess .
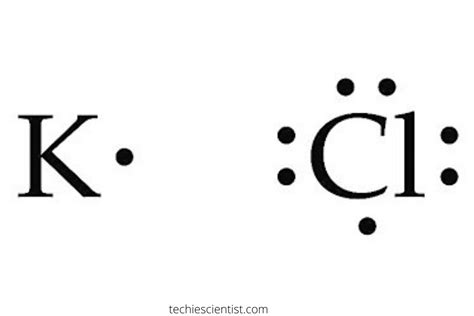
Lewis Structures. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and .Nob 21, 2023 — A Lewis dot structure is a diagram showing how many valence electrons are in an atom or molecule. It shows the covalent bond in molecules, and predicts how atoms are most likely to chemically.Lewis Dot Structures Hun 27, 2022 — A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in .Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. .
May 28, 2024 — The resultant covalent, or molecular, bond is drawn as a line in a two-dimensional picture called a Lewis structure. These structural images are named after Gilbert Lewis, the American chemist who first proposed that covalent molecules could be represented visually. . one electron dot structure for chlorine should be placed in each of these .Cl2 is the diatomic form of chlorine gas, a strong oxidising agent and a greenish-yellow gas. The Lewis dot structure for Cl2 demonstrates how a single covalent bond between the chlorine atoms creates a stable octet of electrons around each Chlorine atom.
Hun 1, 2022 — Calcium chloride lewis dot structure. Lewis dot structure of calcium (Ca) and chlorine (Cl) is a unique structure because it is an ionic compound formed by a metal (Ca) and non-metal chlorine (Cl) The Lewis dot structure of CaCl2 contains a 2+ positive charge on calcium metal and one negative charge on chlorine nonmetal.. As calcium is placed in the .
The most common picture, or model, of elements and compounds used is the Lewis Dot Structure. These pictures show you the type(s) of atom(s) involved, their position in the molecule, and where their valence electrons are situated. . This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas. Exercise .Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination .A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.Ago 5, 2021 — The formal charges being 0 for all of the atoms in the CoCl 2 molecule tells us that the Lewis dot structure presented above is stable.. Thus, the Lewis structure of CoCl 2 is an exception to the octet rule.. Therefore, the Lewis Structure for the CoCl 2 is represented as follows:. CoCl2 Hybridization. To determine the hybridization of Cobalt Dichloride, we first .1 day ago — The molar mass and melting point of beryllium chloride are 79.91 g/mol and 399 °C, respectively. The chemical bonding in Beryllium Chloride is studied by writing down its Lewis structure by following the Lewis approach. After lewis structure, there is a need of understanding its molecular geometry and hybridization of the central atom, Beryllium.May 4, 2013 — A step-by-step explanation of how to draw the Cl2 Lewis Dot Structure (Chlorine gas).For the Cl2 structure use the periodic table to find the total number of.Hul 12, 2023 — Using Lewis Dot Symbols to Describe Covalent Bonding. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. . This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas.19 hours ago — Lewis Structure of Chlorine Dioxide (ClO2-) The Lewis structure is a pictorial representation of valence electrons taking part in the formation of bonds to produce a new molecule with new properties altogether. .Ene 11, 2023 — Some old concepts such as Lewis dot structure and valency are still rather useful in our understanding of the chemical properties of atoms and molecules, and new concepts involving quantum mechanics of chemical bonding interpret modern observations very well. . Each chlorine atom shared the bonding pair of electrons and achieves the electron .Set 2, 2018 — A step-by-step explanation of how to draw the MgCl2 Lewis Dot Structure.For MgCl2 we have an ionic compound and we need to take that into account when we dra.
This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas. Exercise. Write Lewis electron structures for CO 2 and SCl 2, . Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. In the Lewis model, the number of bonds formed by an element in .Mar 8, 2021 — A step-by-step explanation of how to draw the Cl2 Lewis Dot Structure (Diatomic Chlorine).Note that Diatomic Chlorine is often called Molecular Chlorine or j.Using Lewis Dot Symbols to Describe Covalent Bonding. . This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas. Exercise. Write Lewis electron structures for CO 2 and SCl 2, a vile-smelling, unstable .
Share this Doctor: twitter facebook. Dr. Irina Stoica. Optometrist. 1 review # 58 of 893 Optometrists in New York City, New York. Female
chloride lewis dot structure|Lewis Dot Structures